Postgraduates of Biological Chemistry, Biophysics and BioengineeringSociety
Use of the high time resolution technique Fluorescence Correlation Spectroscopy (FCS) to investigate the interactions and mobility of SNARE proteins in cell membranes
Abstract
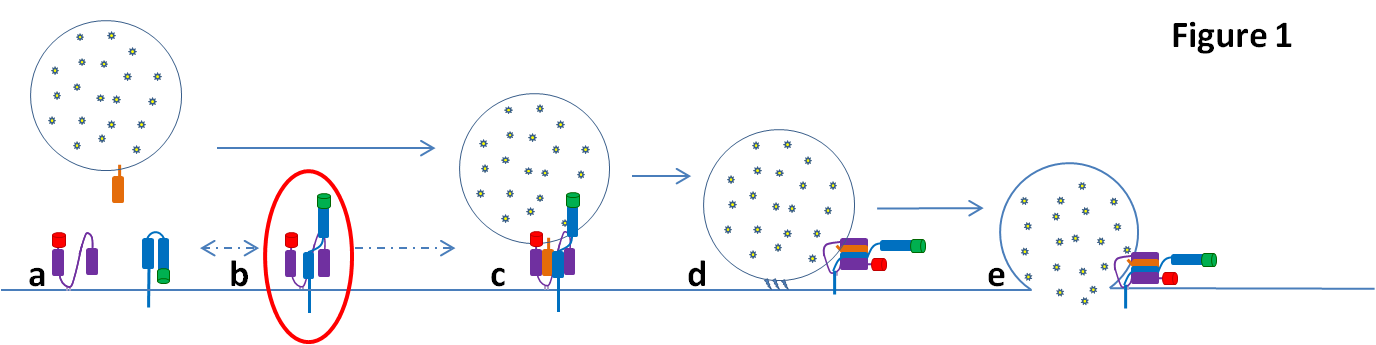
All complex life relies upon communication between billions of cells. A critical component of this is the release of signals by cells for detection by their compatriots. Signal molecules are stored in membrane bound compartments called vesicles and released when the vesicle fuses with the plasma membrane (Figure 1). The protein machinery needed for this fusion is well established, however their temporal and physical organization are poorly understood. SNARE proteins – two target-SNAREs on one vesicular-SNARE (Fig 1a) are brought together (Fig 1c) and ‘zip-up’ to allow lipid mixing between vesicle and membrane (Fig 1d), opening a ‘fusion pore’ for signal release (Fig 1e). One key question is how the tSNAREs are organized before fusion– do they form a binary target complex (Fig 1b), and what regulates it?
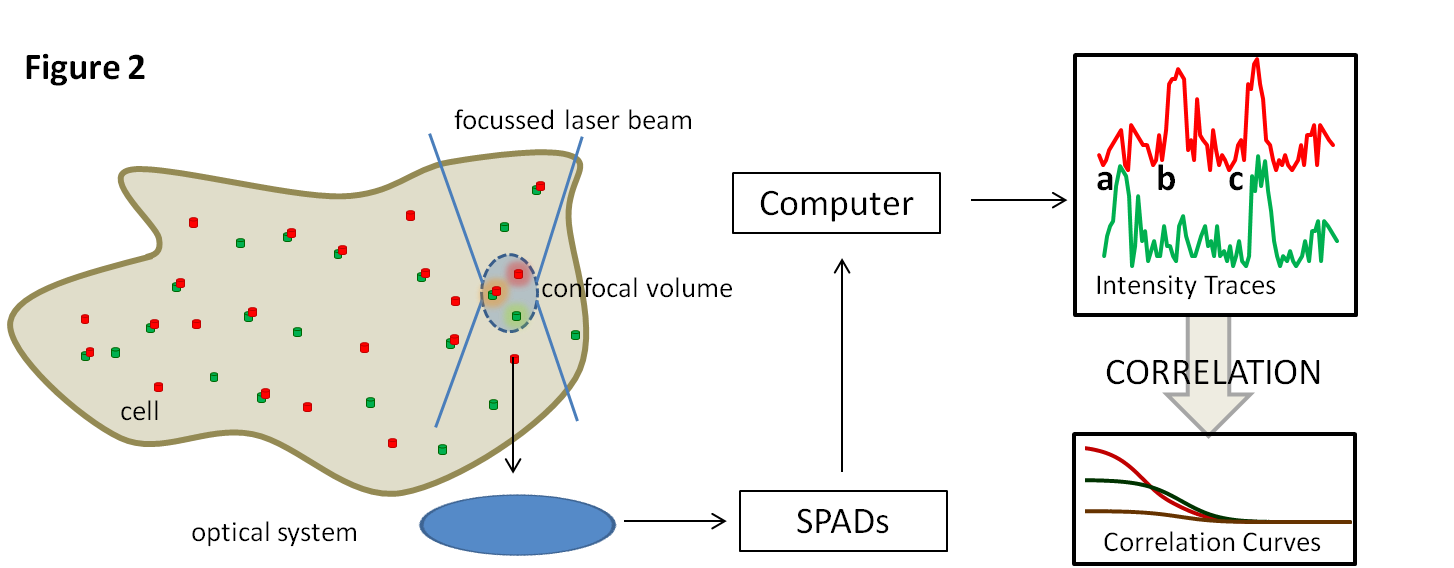
FCS (Figure 2) is an excellent way to answer this question. It allows the measurement of molecular diffusion on a millisecond timescale. Cell membranes containing fluorescently labelled tSNAREs are illuminated at a single point (less than a femtolitre in volume). As fluorophores move in and out of the beam the detected light intensity varies depending on the speed the molecules move at. These characteristics can be extracted through a process of correlation. When red and green labelled tSNAREs interact and move together a peak appears at the same time in both traces (Fig 2c) rather than in one or the other (Fig 2a,b). This provides an assay with which interactions can be monitored in the presence of various other components of the system.